Data Integrity Sop in Pharmaceutical Industry
NEVER mask the original entry. SOP For Batch numbering system.

Sop On Data Integrity In Pharmaceutical Industry
And with the help of trend analysis test results.
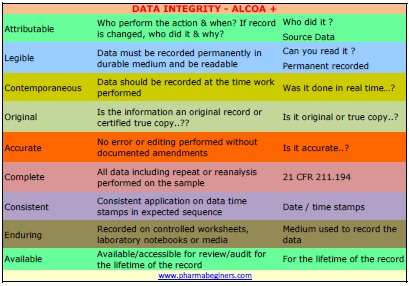
. MHRA -Data Integrity Definitions and Guidance Data Integrity is the extent to which all data are complete consistent and accurate throughout the data lifecycle. Assessments of 200 Singapore-based manufacturing facilities. 10 Data Integrity and Security FAQs - 5th June 2018.
SOP For Operation Cleaning calibration of weighing balance. As global public health and safety standards rise companies must ensure that their employees receive the necessary education and training to remain competitive. 47 Handwritten entries should be made in a clear legible indelible way.
This data collected during batch manufacturing will give precise in depth idea about its fundamentals. 48 Records should be made or completed at the time each action is taken and in such a way that all. They span 12 manufacturing industries ranging from small family-owned Singaporean enterprises to large multinational corporations MNCs whose parent companies originate from 14.
Record writing Data Entry Corrections to written records must be made properly. SOP For Preparation and review of. The audit team shall check for the compliance for the various procedures raw data and record the findings in Internal AuditSelf Inspection Report Annexure-II.
The USFDA Guidance for industry. It will gives all data required for assessing the batch manufacturing on production scale. SOP For Item code generation of raw and packing material.
Without document control software or an EDMS its also difficult to search for documents and drawings in fact on average maintenance engineers and operations teams spend roughly 2 hours. Its comes when first three batches of product being manufactured on production scale very closely. Denotes the type of Audit ie.
A Standard Operating Procedure or SOP is a set of step-by-step instructions compiled by an organization to help workers carry out routine operations in a clear and consistent manner. SOP For Sampling procedure of rinse and swab sample. MHRA guidance for out of specification investigation 2013.
Record writing Data Entry Never use ditto Never use lines with or without arrows to show a continuation of values or entries in a column or row. Various stakeholders contractors and engineers involved in a project can make it difficult to uphold the integrity of the engineering data at hand. Real-time Plant Data Sharing Service WebTechnician Profit-driven Operation Digital Plant Operation Intelligence DPI Quality Stabilization System OpreX Data Model Broker Design Data Validation Plant Information Management System OpreX Batch Solution Secured Remote Solutions Security Program Cybersecurity Consulting Services.
The Manufacturing Transformation Insights Report 2019 analyses data collected from the SIRI. Investigation out of specification OOS test results for pharmaceutical production October 2006. SOP For Product code generation.
Why Pharmaceutical Companies Should Transition to The Cloud - 13th April 2018. SOP For Assigning of manufacturing and expiry date for finished product. NEVER USE correction liquid tape or material.
641 The Auditor shall assign a seven character number as AAXXYY in consultation with QA to Audit report whereAA. Ensure that the instrument used for analysis is calibrated.
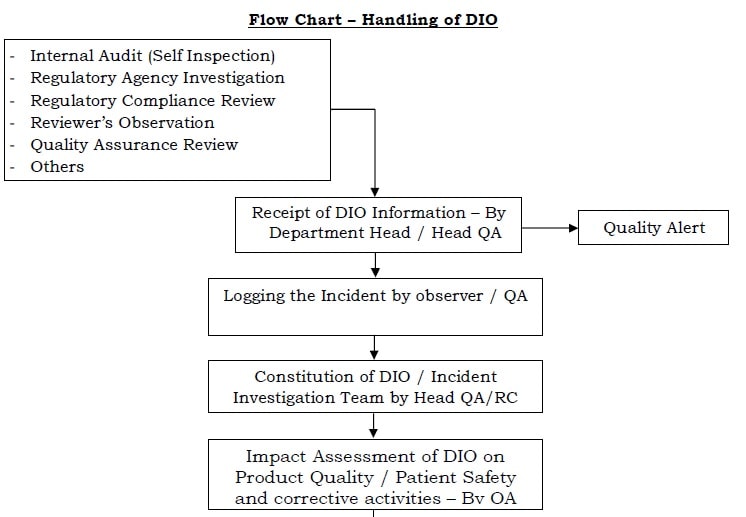
Data Integrity Handling Of Di Observations Dio Pharma Beginners

Data Integrity Handling Of Di Observations Dio Pharma Beginners
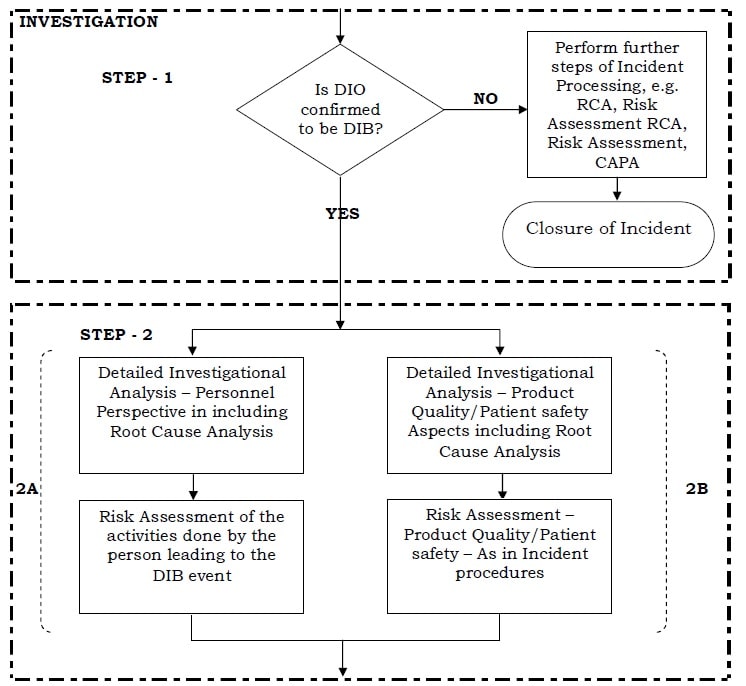
Data Integrity Handling Of Di Observations Dio Pharma Beginners

Comments
Post a Comment